Literature & Publications
|
This poster presentation originally appeared at the American Academy of Periodontology Meeting in Orlando, Florida in November, 2004. |
||||||||||||||||||||||
|
||||||||||||||||||||||
ABSTRACTBackground: In the submerged implant design, the quality of the implant-abutment connection is thought to play a critical role in preserving the alveolar crest bone levels from loss due to oral bacteria. Oral microorganisms that would colonize that structure are suspected to initiate inflammation and promote bone loss over time. |
||||||||||||||||||||||
INTRODUCTIONMicrobial accumulation around dental implants may lead to inflammation and result in a condition known as peri-implantitis. This condition is similar to the one affecting the natural teeth, and supporting structures, when exposed to the microbial challenge. The difference is that natural teeth benefit from the buffering power of the junctional epithelium and periodontal ligament. In the natural dentition, the junctional epithelium provides a seal at the base of the sulcus against the penetration of the bacteria and bacterial by-products. If the seal is destroyed the epithelium migrates apically forming a periodontal pocket. The other natural line of defense absent from the endosseous implant structures is the periodontal ligament. Since there is no cementum or fibers inserted on the surface of an implant, and therefore no periodontal ligament and space, infection will spread directly into the
osseous structures. Peri-implantitis, like periodontitis, if left untreated could result in bone loss and ultimately implant loss. This problem is further compounded by the implants system utilized. Two main dental implant designs have been on the market; submerged and non-submerged. The submerged design necessitates the placement of the coronal portion of the implant at or below the level of the alveolar crest. The non-submerged design requires placing the top of the implant above the level of the alveolar crest. In some of the submerged designs, a “microgap” may exist at the level of the alveolar crest where the abutment and implant body meet. This microgap is usually associated with increased inflammation and alveolar crestal bone loss. One of the prevailing hypothesis regarding this phenomenon is that oral bacteria colonize that area, during surgery or after placement of the abutment, hide and lead to infections over time. The implant well may act as a bacterial reservoir, from which microorganisms may seep in and out perpetuating the infective process that will lead to inflammation and ultimately bone loss. |
||||||||||||||||||||||
MATERIALS & METHODSIn the first phase of the experiment, the ability of the seal to shield the internal well of the implant from outside bacteria was tested. Ten wide body Bicon implants (5x11mm Uncoated Implant 3.0mm well), and 10 abutments (5x6.5mm 0˚ Abutment 3.0mm post) were used. All experiments were carried out in a sterile environment, under a cell culture hood. The abutments were seated on the implant bodies according to the guidelines given by the manufacturer. The implant abutment units (IAU) were then immersed individually in glass culture tubes containing 10 ml of a bacterial mixture ( Actinobacillus actinomycetemcomitans serotype b ATCC strain 43718, Streptococcus oralis ATCC strain 35037 and Fusobacterium nucleatum ATCC strain 10953 at optical density 1) in Brain-Heart infusion broth. The 10 IAUs were incubated for 24 hours in an anaerobic chamber at 37˚C. After that time each glass culture tube was removed from the anaerobic chamber, the bacterial broth discarded, the IAUs washed twice in sterile PBS and fixed with 4% formalin overnight and prepared for scanning electron microscopy (SEM) viewing. At that point the abutments were separated from the implant bodies and the inside of the well was analyzed for bacterial presence by SEM. |
||||||||||||||||||||||
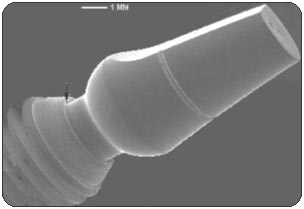
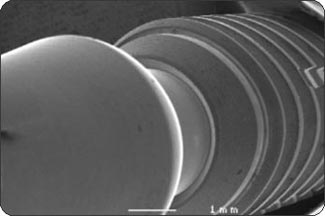
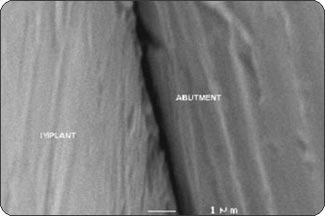
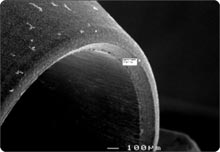
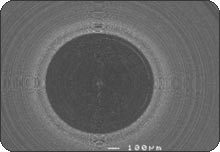
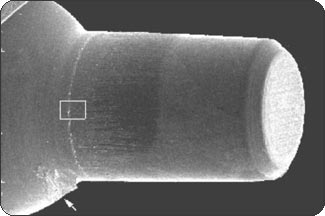
RESULTSPhase 1: Outside-in experiment. The IAUs were tapped in place and examined in the SEM. The overall appearance of the assembled unit was generally clean and free of debris. The abutment/implant interface was examined at high magnification (Fig. 1A) and showed the presence of a small gap between the implant body and the abutment post. This is explained by the presence of a 120 µm wide chamfer present in the coronal portion of the implant (Fig. 2A), that creates a “crevice” when assembled with the abutment post. That “crevice” as seen by SEM is less then 0.5 µm (Fig. 1B) and does not allow any bacterial invasion. Ten assembled units were incubated in bacterial culture broth. After removing the abutment, the separate components were examined by SEM to determine where the penetration of bacteria stopped. In Fig. 1B, the gap between implant and abutment is too small for bacteria to penetrate and they can only adhere to and colonize the coronal chamfer (“crevice”) of the implant as well as all external surfaces under experimental conditions. There was no evidence of bacterial presence into the implant well (Figs. 2A, B, C). This was true for all 10 tested samples. All bacterial presence seem to stop at a certain distance of the implant-abutment junction (Figs. 3A, 3B). It is of interest to notice that the “real” implant abutment junction providing the seal due to the locking taper design is up to 200 µm below the bottom of the crevice, this area is a bacteria free zone. This seal when in place seems to perfectly unify the implant and abutment. The 1.5 degree tapered post of the abutment, locks into the implant with friction. The metal to metal cold welding of the post against the implant wall creates the impenetrable seal. To test this hypothesis three bacterial sizes were used: small (A. actinomycetemcomitan 0.4 ± 0.1 x 1.0 ± 0.4 µm), medium (S. oralis, 2 µm ) and medium-large sizes (F. nucleatum, 0.4-0.7 x 3-10 µm). The SEM photographs show these bacteria colonizing the implants and abutments. None of the 10 tested samples show microbial presence passed the implant/ abutment junction. The inside well of all implants as well as the bottom of the abutments’ taper appear to be free from microorganisms (Fig. 2C). |
||||||||||||||||||||||
|
||||||||||||||||||||||
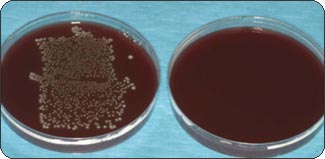
Phase 2: Inside-Out experiment. In the second phase of the experiment, standard microbiology culturing techniques were used to test the resistance of the seal to bacterial seepage. The goal was to assess the capabilities for oral microorganisms to leave the implants’ well and seep out into the environment. All of the 9 test samples (assembled IAUs containing 0.1μl of bacterial gel) showed a clear broth at the end of the 3 days of incubation in an anaerobic chamber at 37˚C. The 3 positive controls (unassembled implant/abutments, with 0.1μl of bacterial gel) showed cloudy broths, which confirmed the viability of the microorganisms (Fig. 4). The last 3 samples (“non-infected” IAUs) that were used to check for microbial cross-contamination (negative controls) during the experiment had a clear broth. From each of the 15 glass culture tubes containing the tests and controls, 20 µl of broth were sampled at 24, 48 and 72 h, and individually plated on TSBY agar. The plates were incubated in an anaerobic chamber at 37˚C for 5 days. The 9 test samples and the 3 negative controls showed no evidence of bacterial presence at 72h as could be seen when looking at the agar culture plates. On the other hand the broth from the 3 positive controls exhibited heavy bacterial presence when plated (Fig. 5).
|
||||||||||||||||||||||
CONCLUSIONSUnder these experimental conditions there was no communication between the inside of the implant and the outside environment. These findings seem encouraging, as they point toward a system (the locking taper) that does not allow oral microorganisms to colonize the implant-abutment interface. This in turn may reduce the possibility of peri-implant inflammation and infection. |